Mission-Critical
Challenges
Are your traditional ways of working resulting in:
Lengthy global approval timelines?
Drug shortages and supply chain disruptions?
Barriers to global market expansion?
There Is A Better Way
ONE PLATFORM. ONE SUBMISSION. ONE CLICK.
The Accumulus platform replaces disconnected systems with a single, cloud-based solution.HOW IT WORKS:
1. Connect Instantly - engage with regulators and stakeholders in real time, eliminating delays and silos2. Exchange Simultaneously - deliver information securely to multiple markets at once with a single click
3. Accelerate Transparently – Collaborate in shared workspaces to enable faster, aligned -decision- making and accelerated access to medicines worldwide
Instant Connection
Simultaneous Submission
Transparent Engagement
CASE STUDY STATISTICS
Reliance Powered by Accumulus
Learn more
1
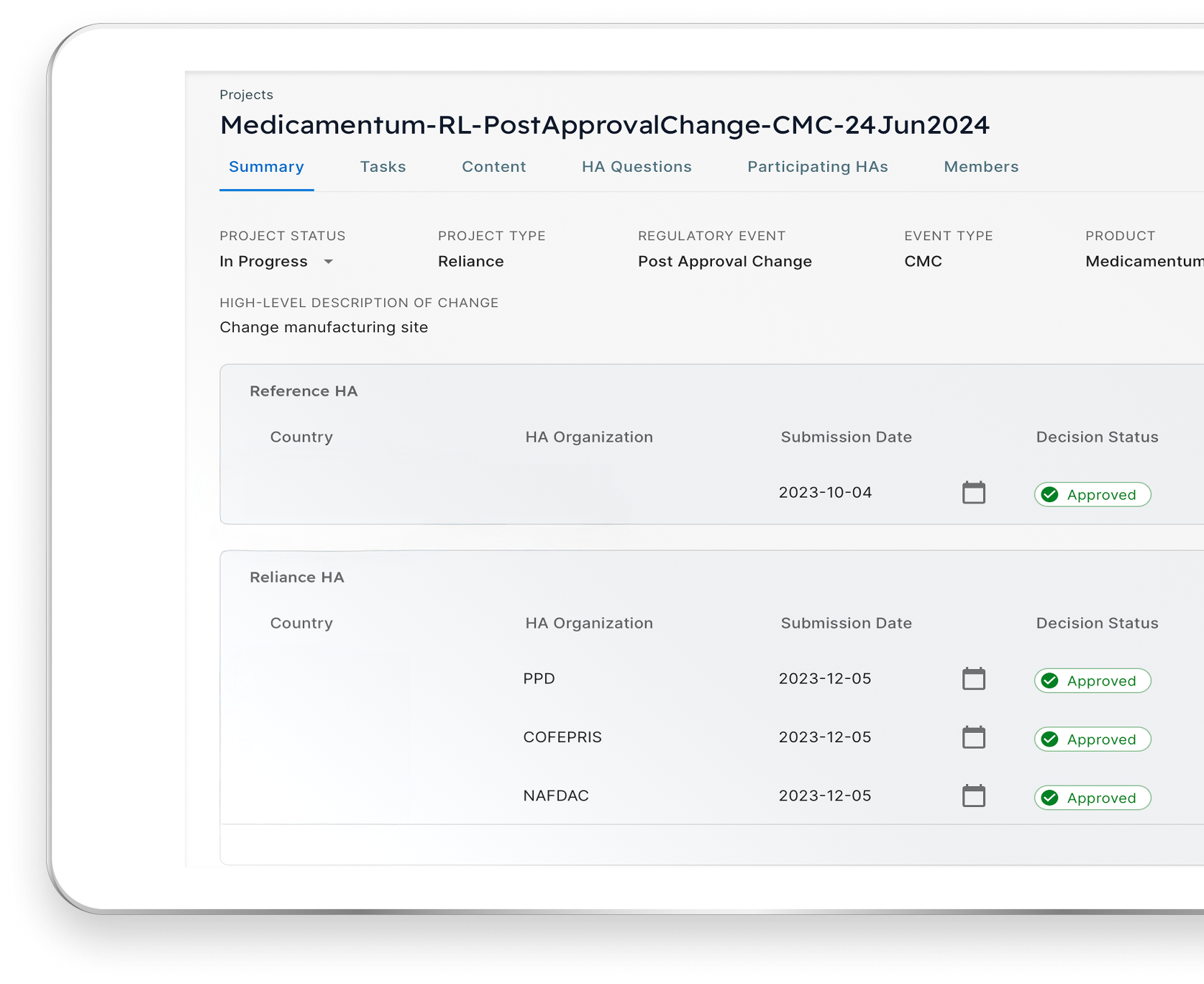
Post-Approval Change

48
Regulators

6
Continents

100
Weeks Saved
REDEFINING WHAT'S POSSIBLE
Experience the
Accumulus Platform Difference
Shorter Global Cycle Times
Transform how information flows between and among industry and regulatory stakeholders to realize shorter cycle times and greater efficiency.
Accelerated Review and Feedback
Streamline collaboration and information exchange to increase availability of the newest and safest versions of products for all populations.
Reduced Administrative Burden
Use automation to drive project timelines, milestones, due dates, advanced tasks, email notifications and more to decrease your administrative burden.
Key Features
Secure, Cloud-Native Architecture
Improves collaboration for users of all regulatory maturity levels through an easily accessible platform.
One Click Information Exchange
Enables collaborative and instant sharing of information, data, insights, and feedback between/among organizations.
Instant Cloud
Upload
Offers full document-based upload with content display for a centralized view of information.
Continuous Data
Streaming
Provides real-time data exchange that enables rapid review and feedback.
Process
Automation
Reduces administrative burden, strengthens operational efficiency, and drives visibility.
Highlights
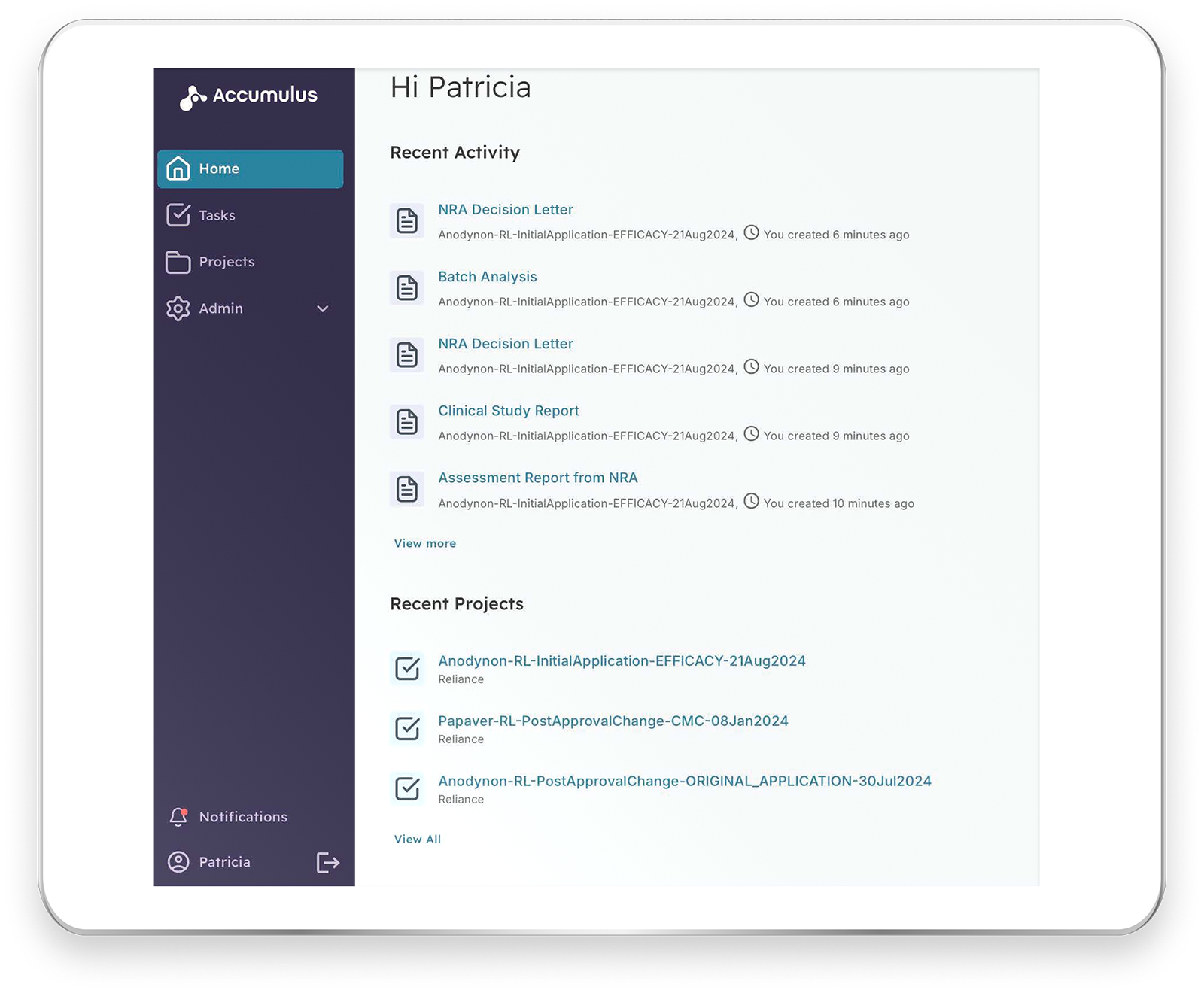
Collaborative Review and Information Exchange
Use secure invitations to invite participants into a collaborative review within the Accumulus platform. Move from a one-one style of review (e.g., one life sciences organization to one national regulatory authority) to a one-to-many approach, reducing review timelines and increasing visibility.
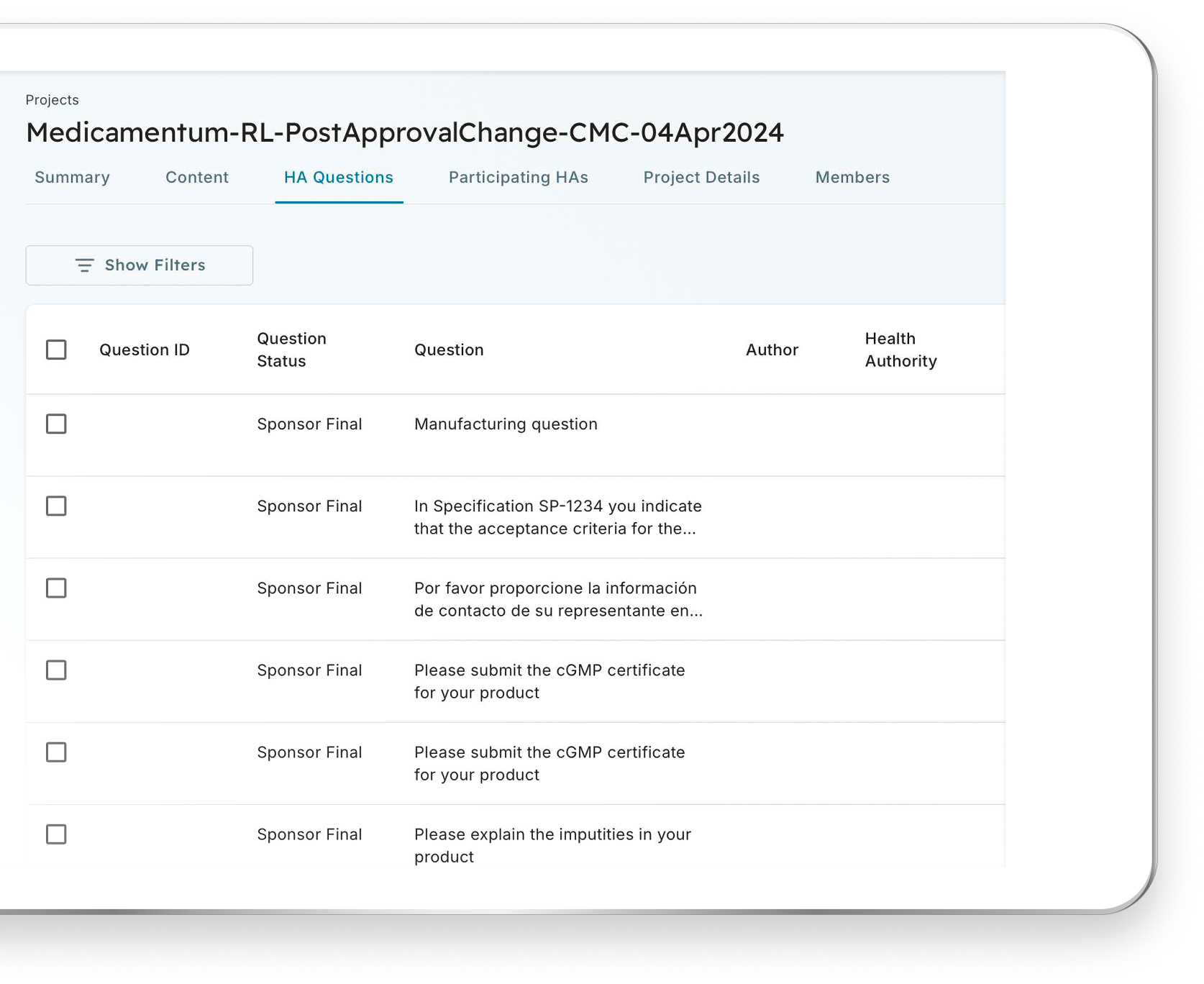
Easy Access to Health Authority Questions and Content
Participate in a shared review to access the same content, questions, and answers as all participants. Regulators can access a shared list of questions for a life sciences organization, and similarly, participating regulators can access responses to questions submitted. The content is organized logically and is fully searchable so that you can find what you need quickly and easily.
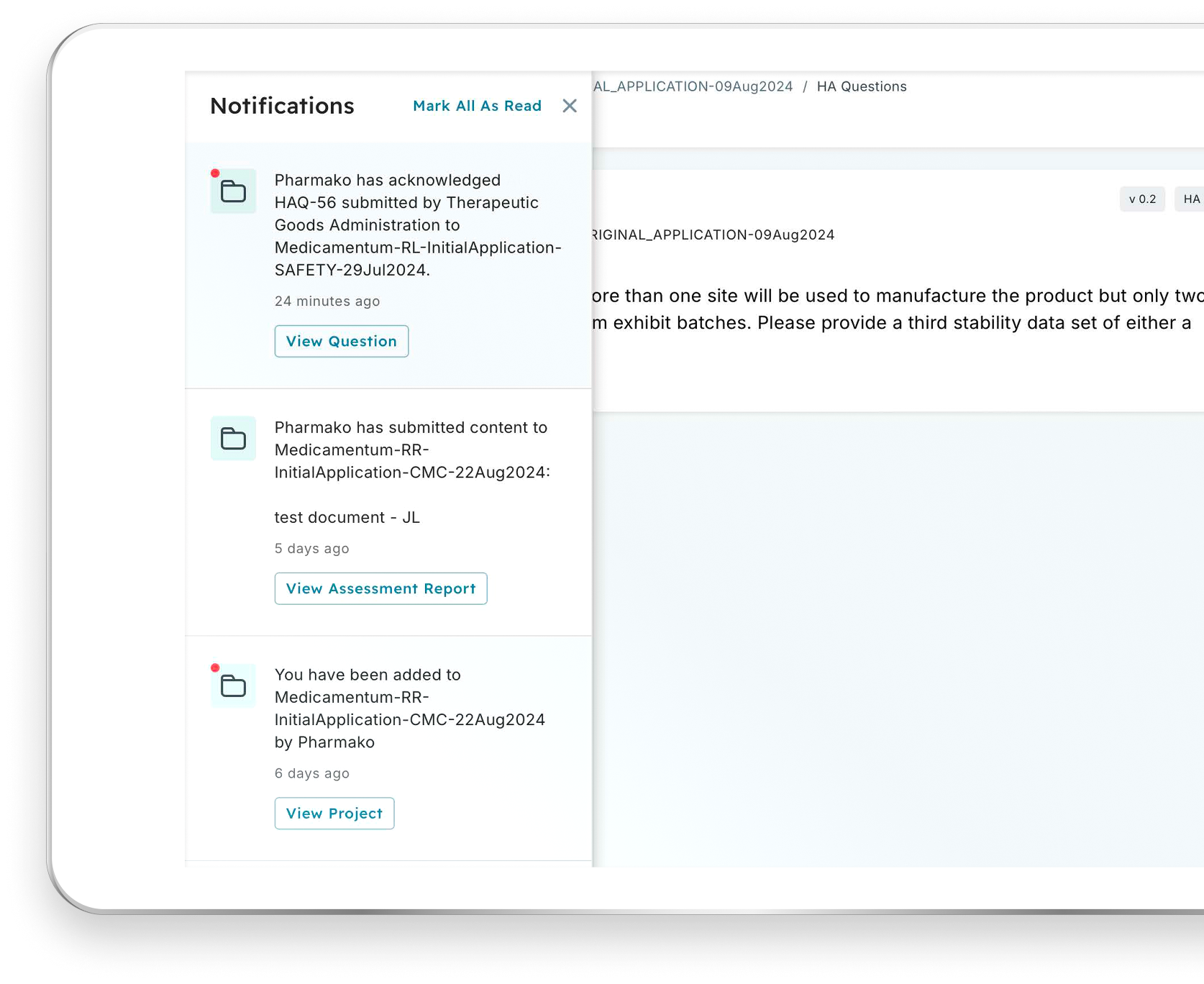
A User Experience Designed for Regulatory Collaboration
Navigate workflows, tasks, and notifications with ease using built-in automation. With input from industry and regulatory subject matter experts, the Accumulus platform user experience is designed to meet your regulatory collaboration needs by simplifying interactions and accelerating results.
SECURITY
Our Security Philosophy
At Accumulus Technologies, safeguarding our users and the data they exchange is our top priority. Our Platform is fortified with cutting-edge SaaS security features, and we maintain constant vigilance by monitoring our cybersecurity defenses 24/7 to ensure the highest level of data protection.

Our Security Philosophy
At Accumulus Technologies, safeguarding our users and the data they exchange is our top priority. Our Platform is fortified with cutting-edge SaaS security features, and we maintain constant vigilance by monitoring our cybersecurity defenses 24/7 to ensure the highest level of data protection.